Label Each Region of the Cooling Curve.
C For section RS of the graph state what is happening to the water molecules as heat is added. By removing the time axis from the curves and replacing it with composition the cooling curves indicate the temperatures of the solidus and liquidus for a given composition.
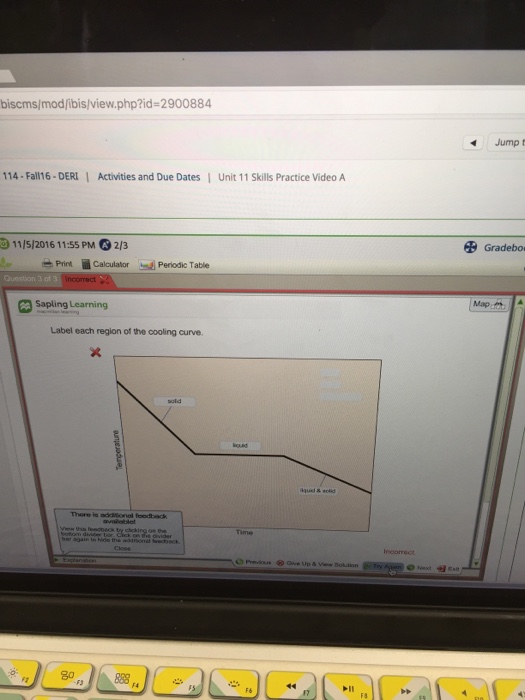
Solved Label Each Region Of Thecooling Curve Please Help Me Chegg Com
B1 pt Draw and label a cooling curve that will result in a microstructure of 100 Fine Pearlite.
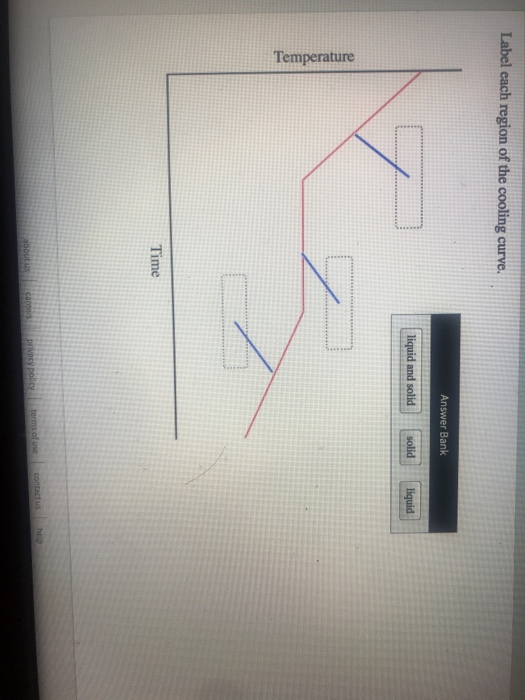
. On the heating curve diagram provided above label each of the following regions. Heating and Cooling Curves. Sketch the simplest phase diagram consistent with.
For example a pressure of 50 kPa and a temperature of 10 C correspond to the region of the diagram labeled ice. Below is an example of a cooling curve used in castings. C1 pt Draw and label a cooling curve that will result in a microstructure of 50 Lower Bainite and 50 Martensite.
This region represents the liquid state water. A1 pt Draw and label a cooling curve that will result in a microstructure of 100 Martensite. Up to 24 cash back 1 Define temperature and heat and give the appropriate unit for each.
Steam above 100C could be steadily cooled down to 100C at which point it would condense to liquid water. There is a gain in both potential energy and kinetic energy therefore the temperature rises and the distance between individual molecules increases and they move faster. Draw a heating curve the heating of a sample of liquid H2O at 200 C to water vapor at 100 C.
Cooling Curve for Pure Metals is shown here. Allow the salol to melt and reach the temperature of the hot water. Cooling Curve A cooling curve is a graphical plot of the changes in temperature with time for a material over the entire temperature range through which it cools.
Variation in hardness with distance from Jominy end is also shown in the diagram. LABEL each of the following of the heating curve of water below. The water could then be cooled to 0C at which point continued cooling would.
Under these conditions water exists only as a solid ice. Boiling point bp and meltingfreezing point mp phase changes meltingfreezing vaporizingcondensing 2. Cooling Curve This is by far the most widely used experimental method.
At the end of the cooling curve phases are shown at room temperature. See the answer See the answer done loading. A pressure of 50 kPa and a temperature of 50 C correspond to the water regionhere water exists only.
For example a pressure of 50 kPa and a temperature of 10 C correspond to the region of the diagram labeled ice. A cooling curve of naphthalene from liquid to solid. I dont know where to begin and what is the concept behind it.
For cooling curve B at T 1 temperature minimum t 1 timing is required to nucleate pearlite as per TTT diagram in Fig. Put the boiling tube in a hot water bath. A pressure of 50 kPa and a temperature of 50 C correspond to the water regionhere water exists only.
Just like heating curves cooling curves have horizontal flat parts where the state changes from gas to liquid or from liquid to solid. Who are the experts. Cooling curves are shown on the temperature log time plot.
This region represents a mixture of liquid and vapour. Under these conditions water exists only as a solid ice. Label the phase regions and draw cooling curves for melts containing 40 K 55 K and 90 K.
The entire experiment could be run in reverse. Im so stuck on it. 2 Describe and graph the temperature changes for a heating or cooling curve and label each part of the curve with the appropriate phases.
Indicate the phases appearing or disappearing at each break or halt. Label each region of the. B For section QR of the graph state what is happening to the water molecules as heat is added.
Circle which phases of water exists in each section of the heating curve. The heating curve for carbon dioxide would have only one plateau at the sublimation temperature of CO 2. Au and Sb melt at 1060C and 630C respectively and form one compound AuSb 3 which melts incongruently at 800C.
Determine the meltingfreezing point and boilingcondensing point from a heating or cooling curve. A cooling curve is a line graph that represents the change of phase of matter typically from a gas to a solid or a liquid to a solid. Be sure to label all axes and label each region of the heating curve.
The red regions indicate where the material is liquid the blue regions indicate where the material is solid and the green regions indicate where the solid and liquid phases are in equilibrium. Take the boiling tube out of the hot water. Cooling Curves Heating curves show how the temperature changes as a substance is heated up.
They show how the temperature changes as a substance is cooled down. Measure and record the temperature of the. It relies on the information obtained from the cooling process.
Cooling curves are the opposite. The independent variable X-axis is time and the dependent variable Y-axis is temperature. Please help me solve this question with an understanding.

Heating And Cooling Curves Also Called Temperature Curves Chemistry For Non Majors

Solved On Sos 2 At A Resting Pulse Rate Of 77 Beats Per Chegg Com
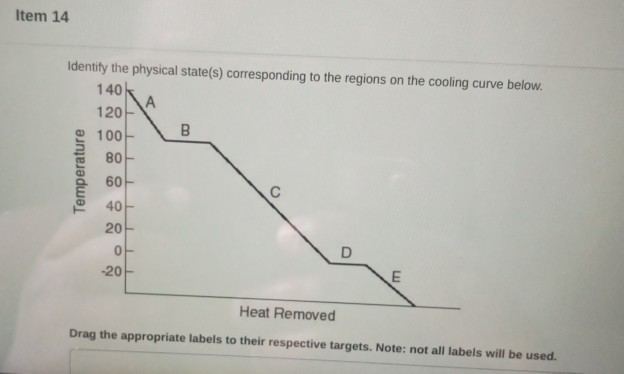
Solved Item 14 Identify The Physical State S Corresponding Chegg Com
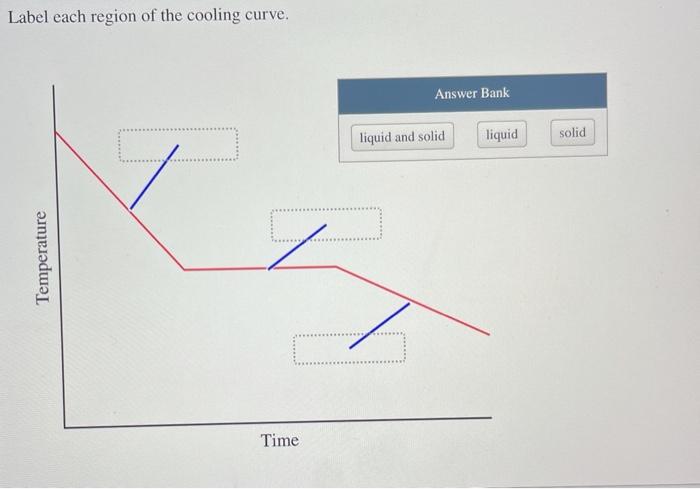
Solved Label Each Region Of The Cooling Curve Answer Bank Chegg Com
No comments for "Label Each Region of the Cooling Curve."
Post a Comment